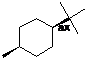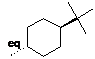![]() Sketch
- Creating and Modifying Models
Sketch
- Creating and Modifying Models
Molecular models in iSpartan are created as standard 2D perspective drawings. Only a few simple tools are needed to create and edit complex drawings.
Tapping on the Sketch icon for the first time brings up a palette of icons and buttons (“Sketch palette”) and a blank white drawing area (“screen”). Drawings are created by tapping atom or group icons in the palette and then tapping or drawing on the screen. Selecting C and drawing CC bonds with your finger or stylus allows you to sketch a complete structural "skeleton" much in the same way you would draw this using pencil and paper. Additional labels, like atom type, stereochemistry, and formal charge, are easily added by tapping the icon that matches the label and then double-tapping the label’s location in the drawing.
There is considerable flexibility in the order in which the skeleton and the labels can be drawn. Also, standard 2D perspective cues can be used to create models with well-defined 3D geometries.
Sketch Palette. The Sketch palette contains tools for making and manipulating drawings. There are also tools for adding cues to designate stereochemistry and set limits for a substructure search.
The palette (below) contains several components: defined atoms, user-definable atom/functional group, common rings, common carbonyl groups, stereochemical markers (one type of marker does not actually appear in the palette, but can be added in drawings as desired), open-site markers, charge/radical markers, and drawing tools.
The palette can be
repositioned anywhere on the screen by dragging it with one
finger. It can also be hidden/displayed by tapping on any![]() .
.
1. Defined atoms. H, B, C, N, O, F, Si, P, Cl, Br and I only.
2. User-definable atom/functional group. This icon is initially designated Ele/Grp. Tap this icon to bring up a keyboard and type a standard chemical symbol. The user-defined symbol can represent an element, e.g., Na or Al, or a standard group, e.g., Ph, OCH3, OMe, NO2, or TMS (about 60 functional group symbols are presently recognized; a list is found under Available Groups). The meaning of this icon can be changed by double-tapping the icon and entering a new symbol. Case is not important, e.g., sn, sN, Sn and SN are all interpreted as Sn.
3. Common
rings. Three icons facilitate the rapid addition of benzene (![]() ), cyclohexane (
), cyclohexane (![]() )
and cyclopentane (
)
and cyclopentane (![]() ) rings to drawings.
) rings to drawings.
4. Common carbonyl
groups. Three icons facilitate the rapid addition of carbonyl (![]() ), carboxylic
acid/ester (
), carboxylic
acid/ester (![]() ) and amide (
) and amide (![]() ) groups to
drawings.
) groups to
drawings.
5. Stereochemical
markers. Wedges and dashes, represented by ![]() and
and ![]() , can be
used to designate in-out stereochemistry. Once a stereochemical marker has been
added to a drawing, it is possible to designate the orientation of hydrogen
atoms and/or substituents bonded to six-member rings as ax(ial) or eq(uatorial)
(ax and eq labels appear only on the drawing, not in the
palette).
, can be
used to designate in-out stereochemistry. Once a stereochemical marker has been
added to a drawing, it is possible to designate the orientation of hydrogen
atoms and/or substituents bonded to six-member rings as ax(ial) or eq(uatorial)
(ax and eq labels appear only on the drawing, not in the
palette).
6. Open-site markers. ![]() designates
an “open-site” in a drawing. Searching the database (or its subset) will return
all structures with any atoms (including hydrogen) or groups in open sites.
designates
an “open-site” in a drawing. Searching the database (or its subset) will return
all structures with any atoms (including hydrogen) or groups in open sites.
7. Charge/radical markers. Conventional bonding rules (neutral C makes 4 bonds, neutral N makes 3 bonds, and so on) are enforced when 2D perspective drawings are converted into 3D models. This is accomplished by adding hydrogen atoms to the drawing so that the bonding rules are obeyed. For example, drawings consisting of only C or N will be converted into 3D models of CH4 and NH3, respectively. (Hydrogen atoms are added to nitrogen, oxygen, phosphorous and sulfur in the 2D drawings.) When another outcome is desired, for example, for an ion or free radical, charge or radical markers must be added to the drawing.
Two icons, ![]() and
and ![]() ,
are used to label atoms that bear formal charges.
,
are used to label atoms that bear formal charges. ![]() is used to label
atoms that are neutral, open-shell radicals. Each of these markers affects the
number of electrons and the number of hydrogen atoms added to the 3D model. For
example, O will produce a 3D model of water, H2O. However,
adding the appropriate marker will result in 3D models of H3O+
(
is used to label
atoms that are neutral, open-shell radicals. Each of these markers affects the
number of electrons and the number of hydrogen atoms added to the 3D model. For
example, O will produce a 3D model of water, H2O. However,
adding the appropriate marker will result in 3D models of H3O+
(![]() ), HO– (
), HO– (![]() ), or HO radical
(
), or HO radical
(![]() ), respectively. (Hydrogen atoms attached
to N, O, P and S will also appear in the 2D drawing.) Only one charge/radical
marker can be assigned to an atom.
), respectively. (Hydrogen atoms attached
to N, O, P and S will also appear in the 2D drawing.) Only one charge/radical
marker can be assigned to an atom.
Only one charge/radical marker is displayed on the palette, but tapping the icon will cause each marker to appear in turn.
8. Drawing
tools. ![]() undoes
the most recent drawing operation.
undoes
the most recent drawing operation. ![]() removes or modifies parts of a drawing.
removes or modifies parts of a drawing. ![]() deletes an
entire drawing (a warning is provided).
deletes an
entire drawing (a warning is provided). ![]() trys to improve the readability of a
drawing by applying various “clean up” procedures.
trys to improve the readability of a
drawing by applying various “clean up” procedures.
back to Sketch – Creating and Modifying Models
How to use Sketch. Sketch is used to create and modify standard 2D perspective drawings. The “skeleton” or network of chemical bonds is drawn by moving a finger (or stylus) across the white portion of the screen in much the same way one draws with paper and pencil. The following terms describe two types of frequently used drawing motions.
Draw. This action combines three actions (touch-drag-lift): one touches the screen where the drawing should begin, drags a finger across the screen to create the desired drawing, and then lifts the finger from the screen to signal the end of the drawing. Draw can be used to add atoms, common rings, and functional groups.
Draw-pause-draw-… . This action combines two or more draw actions to create chains and rings of identical atoms. The lift action that normally ends each draw is replaced by a brief pause in which the finger rests on the screen before dragging in a new direction (see drawing). Lifting the finger after the final draw signals the end of the drawing. Draw-pause-draw can be used to draw chains and multiple bonds as shown below. It can also be used to draw rings (not shown).

· add an atom, ring, or carbonyl group
· replace one atom with another
· define and add a non-standard element or group
· draw one bond “in front of” or “behind” another
· draw 3D perspective with line drawings
· add stereochemical markers (dashes, wedge)
· add an axial or equatorial label to a stereochemical marker (6-member rings only)
· draw Haworth projections (edge-on) rings
· assign charges, unpaired electrons, and the number of “added” hydrogens
· specify “open site” marker(s) for a database substructure search
· adjust a drawing’s appearance (size, screen position, orientation)
· reduce the bond order of a bonds
· remove a stereochemical marker (dash or wedge)
· change or remove an axial or equatorial label from a stereochemical marker)
· remove formal charge & unpaired electron markers
· clear the screen for a new drawing (start over)
· convert a drawing into a 3D model
· save a drawing in the Gallery
· open a drawing from the Gallery
· perform a substructure search on the database
How to start drawing. A drawing can be started in several ways:
1. Tap the icon for an atom (or common ring, or carbonyl group) that is to appear in the drawing. Double tap the screen. Just that atom (or ring, or group) will appear.
2. Draw a CC bond that is to appear in the drawing. You can also use this technique with other atoms. For example, to start with an NN bond, tap N and draw.
3. The drawing technique in #2 can also be used to create chains and rings of identical atoms. To draw an all-carbon chain or ring, draw the first bond, pause briefly without lifting, and then draw the next bond in a new direction. Draw-pause-draw-pause-… can be repeated as many times as you like. The chain will terminate when you lift your finger.
How to add an atom, ring, or carbonyl group. A drawing can be added to in several ways:
1. To add an atom, tap that atom’s icon. Touch the atom in the drawing that will connect to the new atom and draw a bond starting from this location.
2. To add a chain of identical atoms, begin as in #1, then use draw-pause-draw-pause-… to create a chain of bonds. This technique can also be used to add rings of any size to a drawing. Simply draw the last bond in the chain to an atom that already appears in the drawing.
3. To
add a common ring acid/ester, amide or carbonyl group, tap that
ring’s (group’s) icon, touch the atom in the drawing that will
connect to the new ring (group) and draw a bond starting from
this location. The carboxylic acid/ester ![]() and amide
and amide ![]() icons contain an arrow
that shows which atom in these groups will be connected to the existing
drawing. To change the location of this connection point (arrow), tap
the group’s icon until the arrow reaches the desired location.
icons contain an arrow
that shows which atom in these groups will be connected to the existing
drawing. To change the location of this connection point (arrow), tap
the group’s icon until the arrow reaches the desired location.
How to draw multiple bonds. To add a multiple bond, first draw a single bond at the location where the multiple bond is needed, then redraw this line once to make a double bond, and redraw it again to make a triple bond (in other words, touch one end of bond, drag to other end, and lift).
The draw and redraw operations can also be combined and performed as draw-pause-draw…. To draw a double bond, draw a single bond, pause without lifting, then re-draw the bond in the reverse direction. To draw a triple bond, add another pause-draw….
How to fuse 5 and 6-member rings. Tap an icon for the first ring and double tap the screen. Next, tap the icon for the second ring and double tap the bond that the rings will share. This will create a drawing with a fused bicyclic ring system. Note that the (cis or trans) stereochemistry of the ring juncture may be ill-defined.
This technique can also be used to add 5 and 6-member rings to an existing bond in any drawing. Tap the icon for the ring to be added and double tap the bond that will become part of the ring.
How to replace one atom with another. If a drawing contains atom A where atom B is needed, tap the icon for B, then double tap A in the drawing.
This simplifies the drawing of heterocycles. First, draw an all-carbon ring and then replace specific carbons with heteroatoms.
How to define and add a
non-standard element or group. Tap the
icon underneath H and B (this icon will initially be labeled Ele/Grp,
but the label will be replaced each time you specify a new element or group).
To define an element, type the one or two-letter standard
chemical symbol for the element and tap return. To define
a group, type the atoms in the group (e.g., OCH3 or NO2)
or a standard abbreviation for the group (e.g., OMe, Ph, or TMS)
and tap return. Case is not important, e.g., sn, sN,
Sn and SN are all interpreted as Sn. Characters will
appear in the icon in the palette as soon as you type them. Sequences in red
are not recognized (either the group is unknown to iSpartan or
you have not finished typing), whereas recognizable sequences will appear in
green. Tap on ![]() and then on Available Groups to see the
list of available groups.
and then on Available Groups to see the
list of available groups.
The element/group can be added to a drawing, replaced, or removed using the same drawing techniques used for standard atoms. The element/group can also be redefined. Double tap the icon and type the symbol for the new group.
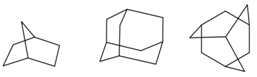
How to draw one bond “in front of” or “behind” another. Conventional perspective drawings use “broken” bonds to show that one bond passes behind another. For example, the broken bond in bicyclo[2.2.0]octane (arrow, left structure) lies behind the vertical CC bond. The same “broken bond” technique is used many times in a fullerene drawing (right structure).

“Broken bonds” are not used in iSpartan. When one bond crosses in front of or behind another, simply draw the new bond across the other. The following drawings are converted into correct 3D models of bicyclo[2.1.0]heptane (left structure) and adamantane (center and right structures), respectively.
How to draw 3D perspective with line drawings. iSpartan attempts to account for intended perspective and orientation when converting 2D drawings into 3D models. The examples in the following table show how this capability can be exploited. Note, that the intended structure might not be realized in every case, and the user should carefully examine the 3D model. More reliable control of stereochemistry and 3D geometry can be achieved using stereochemical markers.
|
2D sketch |
3D model |
|
2D drawing |
3D model |
|
|
cis-2-butene |
|
|
chair cyclohexane |
|
|
trans-2-butene |
|
|
twist boat cyclohexane |
|
|
cis-1,4-dimethylcyclohexane |
|
|
trans-1,4-dimethylcyclohexane |
How to add stereochemical markers (dashes, wedge). 3D information can be added to a drawing by replacing single bonds with stereochemical markers: dashes or wedge. The single bond must be drawn before a marker can be added.
To replace a single bond
with a marker, tap either ![]() or
or ![]() , then re-draw
the single bond. To reverse the orientation of the marker, re-draw
the bond or marker in the opposite direction.
, then re-draw
the single bond. To reverse the orientation of the marker, re-draw
the bond or marker in the opposite direction.
One type of marker can be replaced directly by the other. Tap the desired marker and then re-draw the existing marker.
Markers can also be
converted back into single bonds. Tap ![]() , then double tap
the marker once.
, then double tap
the marker once.
How to add a axial or equatorial marker (6-member rings only). The orientation of a hydrogen/substituent on a 6-member ring can be specified by marking one ring substituent as either ax(ial) or eq(uatorial) (if a molecule contains multiple rings, the conformation of each ring can be specified by marking one substituent per ring). Axial or equatorial markers can only be added to stereochemical markers (dashes, wedge) so the bond connecting the substituent to the ring must be drawn with a stereochemical marker first.
To add an axial or equatorial
marker to a stereochemical marker, tap either ![]() or
or ![]() ,
then double tap the stereochemical marker. ax will appear
on top of the stereochemical marker. To replace ax with eq, double
tap the stereochemical marker again. To remove eq, double
tap the marker again.
,
then double tap the stereochemical marker. ax will appear
on top of the stereochemical marker. To replace ax with eq, double
tap the stereochemical marker again. To remove eq, double
tap the marker again.
Although it is possible to produce a drawing in which several bonds are marked as axial or equatorial, iSpartan uses only one marker when converting a 2D ring drawing into a 3D model. For best results during 2D-to-3D conversion, all substituent bonds to the ring should be drawn with stereochemical markers.
|
2D sketch |
3D model |
|
|
cis-1-tert-butyl-4-methylcyclohexane (axial tert-butyl) |
|
|
trans-1-tert-butyl-4-methylcyclohexane (equatorial methyl) |
How
to draw Haworth projections (edge-on) rings. Haworth
projections are commonly used to represent carbohydrate rings from an “edge-on”
perspective. These projections can be created in iSpartan using
the ![]() stereochemical marker. First, draw
the ring using single bonds. Next, tap
stereochemical marker. First, draw
the ring using single bonds. Next, tap ![]() and re-draw
the two single bonds that appear as wedges in a Haworth projection (see
drawing). The ring bond separating these “wedge bonds” will automatically be
converted into a heavier bond consistent with an “edge-on” perspective. This
perspective will also used to convert the 2D drawing into a 3D model.
and re-draw
the two single bonds that appear as wedges in a Haworth projection (see
drawing). The ring bond separating these “wedge bonds” will automatically be
converted into a heavier bond consistent with an “edge-on” perspective. This
perspective will also used to convert the 2D drawing into a 3D model.
![]()
This method for adding “edge-on” perspective with wedges can be applied to a variety of structures, including acyclic chains. Dashes cannot be used for “edge-on” perspective.
How to assign charges,
unpaired electrons, and the number of “added” hydrogens. Formal
charges and unpaired electrons can be assigned to individual atoms using
charge/unpaired electron markers. To assign a positive formal charge to
an atom, tap ![]() and double tap the atom in
the drawing. To assign a negative formal charge or unpaired electron,
tap the charge/unpaired electron marker until the desired icon
appears (tapping the marker rotates it through three possibilities:
and double tap the atom in
the drawing. To assign a negative formal charge or unpaired electron,
tap the charge/unpaired electron marker until the desired icon
appears (tapping the marker rotates it through three possibilities: ![]() ,
,
![]() , and
, and ![]() ) and double tap
the atom in the drawing.
) and double tap
the atom in the drawing.
The charge/unpaired electron
marker on an atom can be changed in two ways. 1) To replace one marker
with another, tap the desired charge/unpaired electron icon and double
tap the marked atom. 2) To remove a marker, tap ![]() and double
tap the marker.
and double
tap the marker.
Charge/unpaired electron markers play an important role during the conversion of 2D drawings into 3D models in that they determine the number of hydrogens that get added to the model (it is usually unnecessary to draw hydrogens in drawings unless they are needed to mark stereochemistry).
Hydrogens are not shown in 2D perspective drawings unless they have been drawn explicitly (exceptions: hydrogens attached to neutral N, O, P, and S are shown automatically). When a drawing is converted into a 3D model, hydrogens are added to the model according to conventional bonding rules. A neutral carbon atom is assumed to form four bonds, nitrogen three bonds, oxygen two bonds, and so on. Analogous logic is used for atoms that carry a formal charge or unpaired electron marker.
Charge and unpaired electron markers also affect how a 3D model is treated during modeling calculations. The total charge is set equal to the sum of the formal charges in the 2D drawing. A model with one unpaired electron is treated as a free radical (drawings that contain more than one unpaired electron may give unanticipated results).
How
to specify “open site” marker(s) for a database substructure search. To
mark an “open site” for a substructure search on the database, tap
![]() , locate the atom in the drawing that will
carry the open site, and draw a bond starting from this location.
As many open site markers as desired may be added to the drawing.
, locate the atom in the drawing that will
carry the open site, and draw a bond starting from this location.
As many open site markers as desired may be added to the drawing.
To remove an “open site”
marker, tap ![]() , then double tap the ?
marker in the drawing.
, then double tap the ?
marker in the drawing.
Database searches are performed as exact searches when no open sites are marked in the 2D drawing. A “match” is returned only if every atom in the search drawing matches up with the same type of atom in the model in the database. Substructure searches are performed whenever one or more open sites are marked in the search drawing. A “match” can be returned regardless of what atom(s) (including hydrogen) in the database match up with the open site.
How
to undo the last action. Tap ![]() to
return to the drawing as it existed before the last action. Earlier versions of
the drawing cannot be accessed.
to
return to the drawing as it existed before the last action. Earlier versions of
the drawing cannot be accessed.
How to adjust a drawing’s appearance (size, screen position, orientation). All manipulations of 2D drawings involve two-finger gestures. To increase the size of the drawing, spread two fingers over the drawing. To reduce the size, pinch two fingers over the drawing. To move the drawing around the screen, drag two fingers around the screen (left-right or up-down). To rotate the drawing in the plane of the screen, twist two fingers over the drawing.
How
to “clean up” a drawing. Tap ![]() to improve (“clean
up”) the appearance of a 2D drawing and make it easier to read in the Gallery.
Note, that not every “clean up” will produce a desirable result. Tap
to improve (“clean
up”) the appearance of a 2D drawing and make it easier to read in the Gallery.
Note, that not every “clean up” will produce a desirable result. Tap
![]() to undo an unsatisfactory “clean up”.
to undo an unsatisfactory “clean up”.
An additional “clean up”
operation involves a “2D conformation search”. This allows rotation about
single bonds (in 2D) to extend multi-atom chains in an attempt to move atoms to
less crowded positions in the drawing. To access, first, long press
on ![]() and then tap
and then tap ![]() in the
resulting menu. Once again, not every “clean up” will produce a desirable
drawing.
in the
resulting menu. Once again, not every “clean up” will produce a desirable
drawing.
How
to remove an atom or bond. Tap ![]() and then double
tap the atom or bond. Note that if you tap an atom, all bonds to that atom
will also be removed. Removing a bond, either by tapping an atom or by tapping
the bond itself, will also remove terminal atoms, that is, atoms not connected
to any other atoms in the drawing will be removed along with the bond.
and then double
tap the atom or bond. Note that if you tap an atom, all bonds to that atom
will also be removed. Removing a bond, either by tapping an atom or by tapping
the bond itself, will also remove terminal atoms, that is, atoms not connected
to any other atoms in the drawing will be removed along with the bond.
How to reduce the bond order
of a bond. To reduce the bond order of a (multiple)
bond, tap ![]() then double tap the multiple
bond. This reduces the bond order by one. Repeated double taps on a triple bond
will reduce the bond order: triple ® double ®
single ® no
bond.
then double tap the multiple
bond. This reduces the bond order by one. Repeated double taps on a triple bond
will reduce the bond order: triple ® double ®
single ® no
bond.
How
to remove a stereochemical marker (dash or wedge). Tap ![]() , then double
tap the marker. This replaces the marker with a single bond.
, then double
tap the marker. This replaces the marker with a single bond.
How
to change or remove an axial or equatorial label from a stereochemical marker. Tap
either ![]() or
or ![]() , and then double tap
the stereochemical marker where an axial or equatorial label
appears. This cycles the label (in order) among ax (axial), eq
(equatorial) and nothing. The stereochemical marker itself will not be
affected.
, and then double tap
the stereochemical marker where an axial or equatorial label
appears. This cycles the label (in order) among ax (axial), eq
(equatorial) and nothing. The stereochemical marker itself will not be
affected.
How to remove formal charge
& unpaired electron markers. Tap ![]() and double
tap the marker.
and double
tap the marker.
How
to remove “open site” markers. Tap ![]() and
double tap the ? marker in the drawing. Substructure
searches of the database are performed only if one or more “open site” markers
appear in a 2D drawing. Exact searches are the default when a drawing
does not contain open sites.
and
double tap the ? marker in the drawing. Substructure
searches of the database are performed only if one or more “open site” markers
appear in a 2D drawing. Exact searches are the default when a drawing
does not contain open sites.
How
to clear the screen for a new drawing (start over). Tap ![]() .
A warning message will ask you to confirm this operation.
.
A warning message will ask you to confirm this operation.
How to convert a drawing
into a 3D model. Tap ![]() at
the bottom of the screen. The molecular mechanics calculation used to optimize
atom positions (the 3D geometry) may take a few seconds if the model is
relatively large and/or flexible. If the expected delay is longer than a few
seconds, a “progress bar” will appear at the bottom of the screen and a Stop
Minimizer button will appear at the top of the screen. You can tap
the latter to stop the minimizer and provide a 3D structure. In this case, note
that the structure provided is not completely optimized, and there is no way to
resume the molecular mechanics calculation.
at
the bottom of the screen. The molecular mechanics calculation used to optimize
atom positions (the 3D geometry) may take a few seconds if the model is
relatively large and/or flexible. If the expected delay is longer than a few
seconds, a “progress bar” will appear at the bottom of the screen and a Stop
Minimizer button will appear at the top of the screen. You can tap
the latter to stop the minimizer and provide a 3D structure. In this case, note
that the structure provided is not completely optimized, and there is no way to
resume the molecular mechanics calculation.
How
to save a drawing in the Gallery for later use. Tap ![]() at the bottom
of the screen, (optionally) provide a name and tap Save. This
will add the drawing to the Gallery and simultaneously shift the app
into Gallery mode. If you do not provide a name, the molecular formula
will be used, e.g., C2H6O. You can rename the drawing in
the Gallery using the Edit feature.
at the bottom
of the screen, (optionally) provide a name and tap Save. This
will add the drawing to the Gallery and simultaneously shift the app
into Gallery mode. If you do not provide a name, the molecular formula
will be used, e.g., C2H6O. You can rename the drawing in
the Gallery using the Edit feature.
If you tap Don’t Save instead of Save, you can inspect all of the drawings and models in the Gallery, but the current drawing will not be saved in the Gallery.
How
to open a drawing from the Gallery. Tap ![]() ,
locate and double tap on the drawing of interest.
,
locate and double tap on the drawing of interest.
How
to take a photo of a drawing. Tap ![]() at the top right
of the screen and select Save Sketch in Photo Gallery from the menu.
Photos can be accessed using the Photos app.
at the top right
of the screen and select Save Sketch in Photo Gallery from the menu.
Photos can be accessed using the Photos app.
How
to e-mail a drawing. Tap ![]() at the top right of the screen and select e-mail Sketch
from the menu.
at the top right of the screen and select e-mail Sketch
from the menu.
How
to perform a substructure search on the database. (Landscape
mode on the iPad is preferred over portrait mode for this operation.) Following
specification of "open site" markers (see How to specify "open-site"
marker(s) for a database substructure search), tap ![]() at
the bottom of the screen. Searching will begin immediately. To terminate the
search before it has completed, tap on Cancel at the top
right of “Search Hits”. To remove the search, pull
down on the list of search hits. When the search has completed (or
terminated), tap on an entry to see its structure. In landscape
mode, both the list of search hits and structure of the selected molecule are
shown together on screen. To see a different entry, simply tap on it in the
list. In portrait mode (and on the iPhone), the list and structure are not
simultaneously displayed. Tap on an entry to see its structure and on Search
Hits at the top left of the screen to go back to the list of search hits.
Having selected a structure, tap on 3D at the bottom of
the screen to view properties, spectra and graphics for this model.
at
the bottom of the screen. Searching will begin immediately. To terminate the
search before it has completed, tap on Cancel at the top
right of “Search Hits”. To remove the search, pull
down on the list of search hits. When the search has completed (or
terminated), tap on an entry to see its structure. In landscape
mode, both the list of search hits and structure of the selected molecule are
shown together on screen. To see a different entry, simply tap on it in the
list. In portrait mode (and on the iPhone), the list and structure are not
simultaneously displayed. Tap on an entry to see its structure and on Search
Hits at the top left of the screen to go back to the list of search hits.
Having selected a structure, tap on 3D at the bottom of
the screen to view properties, spectra and graphics for this model.